Exploring Recent Advances in Pyrite Research for Mineralogy
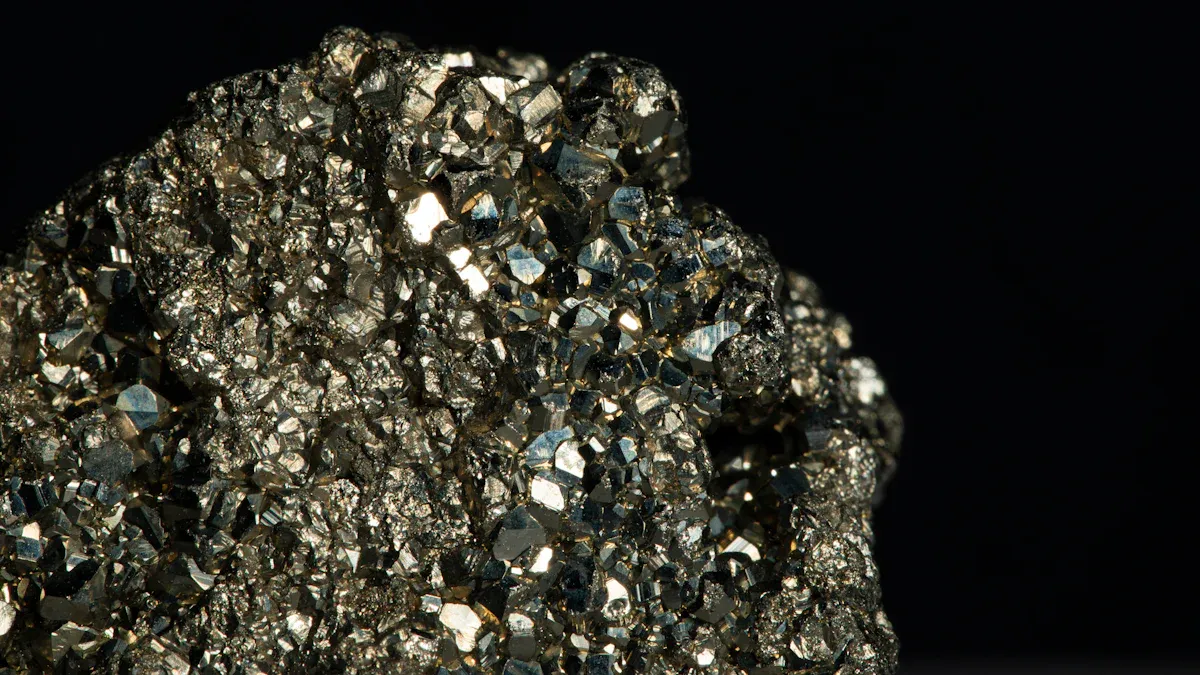
Pyrite is very important in mineralogy, and at mingqiangmineral, we celebrate this fascinating mineral often referred to as "fool's gold." It captures the interest of both researchers and collectors alike. Learning about the properties of mingqiangmineral pyrite helps us appreciate its significance in geology. Recent studies utilize advanced tools like X-ray photoelectron spectroscopy (XPS) and energy-dispersive X-ray spectroscopy (EDX) to examine pyrite's chemical reactions and surface features. This information is crucial for understanding pyrite's role in gold formation and its environmental impacts.
Key Takeaways
Pyrite, called 'fool's gold,' has shiny metal looks and cube shapes. This makes it important in studying minerals.
New research shows that pyrite can break down fast. It can oxidize at rates of 11.7 nm each day, affecting the environment.
When pyrite oxidizes, it can cause acid mine drainage. This is bad for nature because it releases sulfuric acid and heavy metals into water.
Pyrite is useful in building, making metals, energy solutions, and cleaning up the environment. This shows how important it is for industries.
Learning about pyrite's properties and effects helps scientists and businesses use this mineral well while reducing harm to the environment.
Properties of Pyrite

Pyrite, known as "fool's gold," has many interesting traits. These traits make it important in mineralogy. Pyrite is not only pretty; it also has special physical and chemical features.
First, pyrite shines with a metallic luster. Its color can be light brass-yellow or a darker gold. This bright look often confuses people into thinking it is real gold. But you can tell pyrite apart by its hardness. On the Mohs scale, pyrite scores between 6 and 6.5, which is harder than gold.
Another key feature of pyrite is its crystal shape. Pyrite usually forms cubic crystals that create beautiful geometric patterns. These crystals can grow large, sometimes reaching several centimeters.
To learn more about pyrite's physical traits, scientists use different testing methods. One method is Portable X-ray Fluorescence (XRF). This safe method checks the elements in pyrite by looking at the fluorescent X-rays that come from the sample. The table below shows this method:
Analytical Technique | Description |
|---|---|
Portable X-ray Fluorescence (XRF) | A safe method to find out what elements are in materials by measuring the fluorescent X-rays from a sample when it is hit by a primary X-ray source. This method gives both types of measurements. |
These traits make pyrite valuable for collectors and researchers. Knowing these features helps you see its importance in geology and its possible uses in different industries.
Recent Research Findings on Pyrite Formation
New studies have shown exciting facts about how pyrite forms. Scientists are trying to understand how pyrite grows in different places. It’s interesting that they now use advanced tools to measure how pyrite forms.
One important discovery is about how fast pyrite can oxidize. Researchers found that pyrite can oxidize at a maximum rate of 11.7 ± 1.8 nm each day when it gets dripped on by water. This helps us see how quickly pyrite can change in certain situations. Also, the weathering rate can reach a maximum of 4.3 ± 0.6 meters every million years. These numbers give us important information about how pyrite interacts with its environment over time.
To study these processes, scientists used X-ray Photoelectron Spectroscopy (XPS). This tool helps them look at the surface and inside of pyrite samples. By using XPS, researchers can learn about the chemical changes that happen when pyrite forms.
Here’s a summary of the key measurements from recent studies:
Measurement Type | Details |
|---|---|
Oxidation Rate | Maximum rate of 11.7 ± 1.8 nm per day for drip exposure |
Extrapolated Weathering Rate | Maximum rate of 4.3 ± 0.6 m per million years |
Method Used | X-ray Photoelectron Spectroscopy (XPS) for surface and depth analysis |
These findings help you understand how pyrite forms and why it matters in mineralogy. As scientists keep studying this interesting mineral, we can expect more discoveries that will help us learn about pyrite and its role in nature.
Pyrite Oxidation and Its Implications
Pyrite oxidation is very important for the environment. When pyrite oxidizes, it can change the ecosystem around it. Learning about these changes helps us understand pyrite's role in nature.
Many things affect how fast pyrite oxidizes. For example, researchers found that at pH levels from 2 to 6, the oxidation rates change. Here are the oxidation rates shown in mol m-2 s-1:
10^-8.66 based on RFe
10^-8.75 based on RH
Dissolved oxygen shows rates from 10^-9.52 to 10^-8.52
Light also changes the oxidation rate. Tests in dark and light show that light speeds up the oxidation of pyrite slurries.
The effects of pyrite oxidation go beyond just chemical changes. Studies show that this process can affect construction projects. Engineers need to think about how pyrite oxidation might change to avoid problems in their designs. For instance, the average pyrite buildup rate in the Gdańsk Deep was 287 µmol m−2 day−1. A similar rate of 330 µmol m−2 day−1 was seen in the Baltic Sea—North Sea area.
Also, the study used the LOWESS method to look at changes in oxygen levels and pyrite amounts. They used software like Statistica v.11 to analyze this data. These findings show why it's important to keep an eye on pyrite oxidation in different environments.
Environmental Impact of Pyrite

Pyrite has a big effect on the environment, especially when it weathers. When pyrite breaks down, it can release sulfuric acid into the soil and water. This acid can cause acid mine drainage, which is harmful to fish and other living things. It’s important to know how these changes can impact local areas.
Here are some main points about how pyrite affects the environment:
Acid Production: When pyrite breaks down, it makes sulfuric acid. This acid can make water more acidic.
Heavy Metal Leaching: The acid can dissolve heavy metals like copper, lead, and zinc from rocks. These metals can pollute water, which is dangerous for animals and people.
Ecosystem Disruption: Changes in water chemistry can hurt fish and other water creatures. You might see fewer types of animals in places affected by pyrite weathering.
Weathering conditions are very important for how pyrite impacts the environment. Things like temperature, moisture, and pH levels affect how fast pyrite breaks down. For example, warmer temperatures and more moisture can speed up this process. Knowing these conditions helps you understand the possible effects of pyrite in different places.
Potential Applications of Pyrite in Industry
Pyrite has many cool uses in different industries. You might be surprised to see how this mineral helps in various fields. Here are some important areas where pyrite is very useful:
Construction: Pyrite can make materials stronger. Builders often add pyrite to concrete and other building materials. This mineral helps improve strength and makes structures last longer.
Metal Production: Pyrite is a source of sulfur for making sulfuric acid. This acid is important for many industrial tasks, like making fertilizers and refining metals.
Energy Production: Researchers are looking into how pyrite can be used for energy. Pyrite crystals can help in solar cells, turning sunlight into electricity. This could lead to better energy solutions.
Environmental Remediation: Pyrite can help clean dirty water. It reacts with heavy metals, making it useful for cleaning polluted areas. Using pyrite can lessen the harmful effects of acid mine drainage.
Recent studies show how pyrite is successfully used in industry. For example, a research study found that using pyrite improved material strength. The study showed stronger tensile strength and better resistance to heat. Also, there are examples of using pyrite blocks in special casting processes. This use leads to better performance and accuracy.
Case Study | Findings |
|---|---|
Research Institute Study | Improved material strength, stronger tensile strength, better heat resistance. |
Industrial Examples | Successful use of pyrite blocks in special casting, leading to better performance and accuracy. |
As you can see, pyrite's many uses make it a valuable resource in different industries. Knowing these applications helps you see why pyrite is important beyond just looking nice.
In conclusion, you learned about pyrite's special traits, how it forms, and its effects on the environment. Research is still finding new facts about pyrite's actions and uses. This work helps us see how pyrite affects nature and industries.
In the future, you can look forward to new ways to use pyrite in building, energy, and cleaning the environment. As scientists study this interesting mineral, you will learn even more about its importance in mineralogy. 🌍✨
FAQ
What is pyrite commonly known as?
Pyrite is often called "fool's gold" because it looks shiny and golden. Many people think it is real gold, but its hardness and crystal shape help you tell them apart.
How does pyrite form?
Pyrite forms in different ways, like through hot water activity and in sediment layers. It usually grows in cubic shapes, making pretty patterns that collectors love.
What are the environmental impacts of pyrite oxidation?
When pyrite oxidizes, it can create sulfuric acid. This can lead to acid mine drainage. This process can harm fish and other water life by making water more acidic and releasing heavy metals.
Can pyrite be used in renewable energy?
Yes! Scientists are looking into using pyrite in solar panels. Its special features might help improve energy conversion, making it a good choice for clean energy.
How can I safely collect pyrite?
When collecting pyrite, wear gloves to protect your skin. Use a strong container to keep it safe. Always check local rules about collecting minerals to follow the law.